Review Article
Tuberculosis: Recapitulation of an ancient foe
Kanchan Srivastava, Apoorva Narain, Surya Kant
Department of Respiratory Medicine, KGMU, UP, Lucknow, India, 226003
Correspondence: Surya Kant, email: skantpulmed@gmail.com
Keywords: Tuberculosis, Mycobacterium tuberculosis, Drug resistance, BCG
Received: 25/10/2019; revised 20/11/2019; Accepted: 27/11/2019
[Citation: Srivastava, Kanchan., Narain A,poorva., & Kant, Surya. (2019). Tuberculosis: Recapitulation of an ancient foe. DHH; 6(4):http://www.journalofhealth.co.nz/?page_id=1946].
Abstract
Tuberculosis is a communicable disease that is a major cause of ill health, one of the top 10 causes of death worldwide and the leading cause of death from a single infectious agent (ranking above HIV/AIDS). It is caused by the bacillus Mycobacterium tuberculosis (Mtb), which is spread when people who are sick with TB expel bacteria into the air; for example, by coughing, laughing and singing. It typically affects the lungs (pulmonary TB) but can also affect other sites (extrapulmonary TB). About a quarter of the world’s population is infected with Mtb and thus at risk of developing TB disease. With timely diagnosis and treatment with first-line antibiotics for 6 months, most people who develop TB can be cured and onward transmission of infection curtailed. The number of TB cases occurring each year can also be driven down by reducing the prevalence of health-related risk factors for TB (smoking, diabetes and HIV infection), providing preventive treatment to people with a latent TB infection, and taking multisectoral action on broader determinants of TB infection and disease (e.g. poverty, housing quality and undernutrition). The Sustainable Development Goals (SDGs) include ending the TB epidemic by 2030. The End TB Strategy defines milestones (for 2020 and 2025) and targets (for 2030 and 2035) for reductions in TB cases and deaths. The Honorable Indian Prime Minister has committed to eliminating TB in India by 2025, five years before the global target.
Introduction
Mycobacterium tuberculosis (Mtb) is for sure the most successful conqueror of the human lives as it infects one in three people worldwide every year on a global scale. Nearly 9 million new TB cases add to the TB burden of our planet annually. Thought to be one of the oldest human diseases, over the years not only the medical implications but also the social and economic impact of TB has been enormous. Globally, an estimated 10.0 million (range, 9.0–11.1 million) people fell ill with TB in 2018, a number that has been relatively stable in recent years. The burden of the disease varies enormously among countries (Fig 1). TB affects people of both sexes in all age groups but the highest burden is in men (aged ≥15 years), who accounted for 57% of all TB cases in 2018 (1, 2).
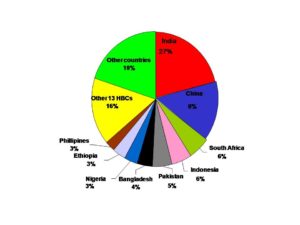
Fig 1. The burden of TB disease among countries (HBC = High Burden Country)
The word “tuberculosis” is a derivative of the Latin world ‘tubercula’ which means of ‘A small lump’ (3, 4, 5). Several names have been used to refer to TB. Pulmonary TB has been referred to as “tabes pumonali”. Cutaneous tuberculosis has been called “lupus vulgaris”, Abdominal tuberculosis has been called “tabes Mesenterica” Acute progressive TB has called “galloping tuberculosis” (6). TB or Kshaya rog or “The Great White Plague” is caused by an airborne infectious agent, Mtb. It is an ancient disease and continues to be the second largest infectious killer worldwide after HIV. TB is thought to be introduced into humankind with the domestication of the cattle around 5000 BC. The causative agent of human TB is very similar to the bacteria which cause TB in cattle called Mycobacterium Bovis. The signs of TB have also been seen in the fragments of a spinal column of Egyptian mummies of 2400 BCE (7).
History of TB:
TB was epidemic in Europe and the US in the 1600s, probably started in the 17th century and lasted two-hundred years, was known as the “Great White Plague”. The high population density, as well as the poor sanitary conditions, created a perfect environment for the propagation of the disease (6). During Pre-Columbian America, In South America, the first evidence of the disease is found in the Arawak culture around 1050 BCE (8). Although the most significant finding belongs to the mummy of an 8 to 10-year-old Nascan child from Hacienda Agua Sala, dated to 700 CE. In Europe, no significant advances were made regarding TB during the middle ages, but, in a document from the 12th century, it is recorded that the pagans believed that TB occurred when a dog-shaped demon occupied the person’s body and started to eat his lungs. (9).
With the spread of disease, monarchs were seen as religious figures with magical or curative powers. It was believed that Royal Touch, the touch of the sovereign of England or France, could cure diseases due to the divine right of sovereigns (5). This practice was so common in France, that scrofula became known as the “mal du roi” or the “King’s Evil”.
The first evidence of the infection in humans was found in a cemetery near Heidelberg, in the neolithic bone remains of the body of a mummy that show evidence of the type of angulations often seen with spinal tuberculosis (10).
The first mention of TB in Chinese literature appears in a medical text written by Emperor Shennong of China (2700 B.C.) (11) The Yellow Emperor, Huangdi Neijing, describes xulao bing (weak consumptive disease), which is believed to be TB. He describes the persistent cough, abnormal appearance, fever, a weak and fast pulse, chest obstructions, and shortness of breath (12). Girolamo Fracastoro became the first person to propose, in his work De contagione, that phthisis was transmitted by an invisible virus, usually transmitted through direct contact or the discharged fluids of the infected, what he called fomes. Franciscus Sylvius, a Dutch physician, and scientist began differentiating between the various forms of TB (pulmonary, ganglion). His book, Opera Medica, published posthumously in 1679, depicted the exact pathological and anatomical features of the disease. He was the first person to recognize that the skin ulcers caused by scrofula resembled tubercles seen in phthisis, (13) noting that “phthisis is the scrofula of the lung”. Richard Morton published Phthisiologia, seu exercitationes de Phthisi tribus libris comprehensae in 1689, in which he emphasized the tubercle as the true cause of the disease. In 1720, the English physician Benjamin Marten surmised for the first time, that TB could be caused by “wonderfully minute living creatures” or some type of Animalcule. Marten’s theory was rejected and it took another 162 years before Robert Koch demonstrated, it to be true. Robert Whytt (1768) gave the first clinical description of tuberculosis meningitis (14). Gaspard Laurent Bayle (1774-1816) introduced the term “Tuberculosis” for the first time. The incidence of TB grew progressively during the middle ages, peaking between the 18th and 19th centuries.
There have been references to TB in several works of fiction. Such as the” consumptive lover” of Much Ado about Nothing in William Shakespeare’s plays and “Scrofula” in Macbeth. Thomas Mann’s The Magic Mountain contains one of the most well-known descriptions of TB Sanatorium.
According to the World Health Organization (WHO), every third individual of the human population is latently infected with TB bacteria. The infection of TB without disease symptoms or progression of pathological conditions is called latent TB infection. However, latent infections may turn active in 10% of cases and develop into active disease.
Battle with tuberculosis
In the battle against TB, the first and foremost step had been the introduction of sanatorium cure in the late nineteenth and twentieth century in the pre-antibiotic era. Sanatoria were the health resorts where the TB patients used to keep so that they could get good nutrition, fresh air, and sunlight.
Containment
The scientific advances – Robert Koch, a Prussian physician, discovered the cause of TB. Villemin’s experiments had confirmed the contagious nature of the disease and had forced the medical community to accept that tuberculosis was indeed an infectious disease, transmitted by some etiological agent of unknown origin. In 1882, Robert Koch utilized a new staining method and applied it to the sputum of TB patients, revealing for the first time the causal agent of the disease: Mycobacterium tuberculosis, or Koch’s bacillus (16). He was awarded a Noble Prize in Physiology and Medicine in 1905. This disease poses a tough challenge in the developing countries, which accounts for about 95% of all TB cases. South-east Asia, Western-pacific and Africa are the most severely hit regions.
The journey of Mycobacterium tuberculosis
Mtb is a microscopic, slow-growing, rod-shaped bacterium that is transmitted through the air when the infected person coughs, spits, laughs or sneezes. Clinically, tuberculosis is categorized into:-
Primary TB – The first infection with TB bacilli, which gears the body’s immune systems to either eliminate the pathogen fully or prevent the spread of bacteria and the progression of the disease.
Secondary reactivated TB- Occurs in people with a weakened immune system. In this case, the TB bacilli suddenly awake inside a lung tubercle and reactivate to rupture the tubercle and spread through the lungs.
Drugs to combat TB
During the 19th century, bed rest and changed environment emerged as important forms of treatment of TB. The antibacterial drugs such as sulfonamide and penicillin had been discovered in the 1930s and chemotherapy for infectious diseases had been started, but these antibacterial agents were ineffective against Mtb. In 1882 Robert Koch discovered the microbial causes of TB disease which revolutionized the development of vaccine and effective drugs to combat TB (17). In 1943 Streptomycin was successfully purified from soil bacteria Streptomyces griseus. Streptomycin had excellent inhibitory activity against Mtb with comparatively low toxicity to humans and animals. The first administration of the antibiotic in critically ill TB patients was done in 1944. (18, 19). The fortuitous discovery of two other drugs, para-aminosalicylic acid (PAS) and Isoniazid (1952) brought the hope for a cure (20). Chemotherapy of TB underwent revolutionary changes in the seventies owing to the availability of two well-tolerated and highly effective drugs – Rifampicin and Pyrazinamide. These drugs allowed short-course chemotherapy (SCC) and made it possible to simplify treatment and reduce its duration.
MDR-TB takes longer to treat and can be cured with second-line drugs, which are more expensive and have more serious side effects. The misuse of these, second-line drugs results in drug-resistant TB (XDR-TB) and total-drug-resistant TB (TDR-TB). Modern anti-TB treatment can take a long time but can be effective. As a result, depending on patients’ circumstances, the treatment may often be interrupted.
The success of streptomycin monotherapy (one drug treatment) did not last long, the resistant mycobacterial strains began to appear. A rapid introduction of new antibiotics was important to outpace the problem of emerging drug resistance. In subsequent years, a large number of antibiotics had been introduced in the clinical practice for the management of TB epidemics. To overcome the problem of drug resistance, streptomycin monotherapy was replaced by multidrug therapy.
A number of medications are being studied for MDR-TB including Bedaquiline and Delamanid. Bedaquiline received U.S. Food and Drug Administration (FDA) approval in late 2012 (17). The safety and effectiveness of these new agents are still uncertain because they are based on the results of relatively small studies. However, existing data suggest that Bedaquiline-based treatment regimens were associated with a large reduction in mortality in patients with drug-resistant tuberculosis, compared with the standard regimen (21, 22, 23).
Vaccine
As of August 2019, there were 23 drugs, various combination regimens and 14 vaccine candidates in clinical trials. The first effective vaccine was developed in 1921 by French bacteriologists Albert Calmette and Guerin, using a live, weakened strain of the bovine tubercle bacillus called M. Bovis responsible for causing TB in cattle. Among the various efforts to design new vaccine against TB, the most clinically advanced vaccine candidates are – MVA65A, a new vaccine developed by the South African Tuberculosis Vaccine Initiative (SATVI), and the recently developed M72/AS01E vaccine which was found to be protective against TB disease in a Phase II b trial among individuals with evidence of latent TB infection (2).
Drug-resistant TB (DR TB)
The political declaration at the UN high-level meeting on TB included commitments to improve the coverage and quality of diagnosis, treatment and care for people with DR TB. Detection of MDR/RR-TB requires bacteriological confirmation of TB and testing for drug resistance using rapid molecular tests, culture methods or sequencing technologies. Treatment requires a course of second-line drugs for at least 9 months and up to 20 months, supported by counseling and monitoring for adverse events. There was some progress in testing, detection, and treatment of MDR/RR-TB between 2017 and 2018. Ten countries accounted for 75% of the global gap between treatment enrolments and the estimated number of new cases of MDR/RR-TB in 2018. Closing the wide gap requires one or more of the following to be increased: detection of TB cases, the proportion of TB cases bacteriologically confirmed, coverage of testing for drug resistance among bacteriologically confirmed cases and coverage of treatment for those diagnosed with MDR/ RR-TB (1, 2).
Resurgence of Tuberculosis
The discoveries by Koch, the introduction of an effective vaccine, and antibiotics during the 1940s to 1960s in TB medication led to the belief that the disease was almost eradicated. However, the belief was dispelled by the worldwide reports of the cases of MDR- TB. After a phase of rapid decline, WHO declared TB a global emergency in 1993. The communities living in poverty with limited access to healthcare facilities, often suffering from malnutrition have been the major targets of TB. Along with several socioeconomic factors responsible for the return of TB, the emergence of drug resistance, the association of TB with the immunosuppressive syndromes such as human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), and lifestyle disorders such as diabetes have been identified as the major reasons.
The outbreak of MDR TB
The emergence of multidrug resistance in 1982 alarmed the world for a worse situation than ever before. Unfortunately, antibiotics acted as a double-edged sword. The antibiotics not only killed the susceptible bacteria but also selected the resistant strains. The emergence of drug resistance Mtb created a severe healthcare challenge indiscriminately for both rich and poor economies of the world. There are two reasons for developing resistance in people are either the person doesn’t take their drugs properly or they get the infection from someone who has already got DR- TB. Moreover, the association of mycobacteria in spreading epidemics of HIV-AIDS and diabetes has exacerbated the problem several folds (27-29). Lack of medication compliance may have contributed to the drug-resistance TB.
Collapse of treatment
There are a number of factors, in addition to possible medication non-compliance, that may lead to the unsuccessful treatment of TB, e.g. failing to diagnose drug resistance TB. Directly Observed Treatment Short-course (DOTS) and DOTS Plus are TB control strategy recommended by WHO. DOTS has been perceived as a viable methodology for treating TB. DOTS contain five fundamental components, 1. Maintained political and fiscal commitment, 2. Examination by sputum smears microscopy, 3. Standardized short-course treatment directly observed by trained healthcare workers. 4. An uninterrupted supply of anti-TB drugs and, 5. Standardized recording and reporting of the results of treatment. Under DOTS, medication is administered by a DOTS agent who is usually a volunteer from the patient’s community and maybe a family member.
Other Socioeconomic factors
Despite the sustained political intervention, financial obligation, research, and community efforts, TB remains a significant health concern around the world. The social issues such as poverty, overcrowding, biomass fuel, smoking, poor ventilation, and malnutrition majorly contribute to the incidence and prevalence of TB. Low-income countries and underprivileged societies, within urban cities in developed countries, present the elevated incidences of TB and associated death rates. Moreover, the other social issues including immigration, social inequalities, and drug or alcohol abuse are also strongly associated with TB. The development of strategies that tackle social, economic and environmental issues such as poverty, food insecurity, drug abuse, public awareness, and empowerment, will immensely benefit tuberculosis care and prevention (30- 35).
TB prevention services
The main health care intervention available to reduce the risk of a latent TB infection progressing to active TB disease is TB preventive treatment. Vaccination of children with the BCG vaccine can also provide protection against TB. In 2018, 153 countries reported providing BCG vaccination as a standard part of childhood immunization programmes, of which 113 reported coverage of ≥90%. WHO guidance issued in 2018 recommends TB preventive treatment for “People Living with HIV/AIDS” (PLHIV), household contacts of bacteriologically confirmed pulmonary TB cases and clinical risk groups. WHO has been intensifying its efforts to support countries in accelerating the TB response, with the engagement of all stakeholders. Actions taken in the past year include high-level missions to countries to optimize the national response; the development and roll-out of new guidelines, roadmaps, and tools; the implementation of the WHO Director-General’s Flagship initiative, “Find. Treat. All. #EndTB”, undertaken jointly with the Global Fund and the Stop TB Partnership; strengthened collaboration with civil society; and implementation of a multisectoral accountability framework for TB to drive sustained action across all sectors. Substantial scale-up will be needed to reach the targets set at the UN high-level meeting (34).
Conclusion:
To meet the challenge of controlling and eradicating MDR-TB and XDR-TB worldwide, substantial monetary investments and extensive human resources development are required. Among the response priorities, rapid detection of anti-tuberculosis drug resistance, the use of appropriate regimens for treatment, and new drug development are of paramount importance. Improved public awareness, up to date and appropriate information and data are powerful weapons in the fight against TB. Furthermore, the strengthening of the current TB control programs globally should continue to be maintained.
Reference
- World Health Organization. Global Tuberculosis Report Geneva: (2018) World Health Organization. Available from: http://www.who.int/tb/ publications/global report/en/2018.
- World Health Organization. Global Tuberculosis Report Geneva: (2019) World Health Organization. Available from: http://www.who.int/tb/ publications/global report/en/2019
- Flick, LF. Development of our knowledge of tuberculosis.Philadelphia, Wickersham;1925.
- Dubos, R, Dubos J. The White Plague, Tuberculosis, Man and Society. Boston; Little, Brown and Company,1952.
- Waksman, Selman A. (1964). The Conquest of Tuberculosis. University of California Press Berkeley and Los Angeles.
- Rubin, SA. Tuberculosis, The captain of all these men of death. Radiol Clin North Am 1995;33; 619-39.
- Zink A, Sola C, Reischl U, Grabner W, Rastogi N, Wolf H, Nerlich A (2003). “Characterization of Mycobacterium tuberculosis complex DNAs from Egyptian mummies.
- Shorter, Edward (1991). Doctors and Their Patients: A Social History. Transaction Publishers. ISBN088738871X. 21
- Shryock, Richard Harrison (1988). National Tuberculosis Association, 1904-1954: A Study of the Voluntary Health Movement in the United States. Ayer Publishing. ISBN0405098316.
- Barnes, David S. (1995). The Making of a Social Disease: Tuberculosis in Nineteenth-century France. University of California Press. ISBN0520087720.
- Elvin, Mark; Cuirong Liu; Tsʻui-jung Liu (1998). Sediments of Time: Environment and Society in Chinese History. Cambridge University Press.
- Chalke HD (1959). Some historical aspects of tuberculosis. Public Health 74: 83–95. doi:1016/S0033-3506(59)80055-X. PMID13809031.
- Comstock G (1994). “The International Tuberculosis Campaign: a pioneering venture in mass vaccination and research”. Clin Infect Dis 19: 528–40. doi:1093/clinids/19.3.528. PMID7811874.
- McClelland C (1909). “Galen on Tuberculosis”. The Physician and Surgeon (Detroit and Ann Arbor: Keating and Bryant) 31: 400–404.
- Yang, Shou-Zhong; Bob Flaws (1998). The Divine Farmer’s Materia Medica: A Translation of the Shen Nong Ben Cao Jing. Blue Poppy Enterprises, Inc.
- Sakula, A. Robert Koch;(1983), Centenarian of the discovery of the tubercle bacillus.1882.Thorax; 37;246-51
- Koch R (10 April 1882). Die Ätiologie der Tuberculose”. Berliner Klinischen Wochenschrift 15: 221–230.32
- McDermott, W, Hadley S.J, Hult Smith H, Tracy A. Streptomycin in the treatment of tuberculosis in humans. Ann Intern Medicine 1947; 27: 769-822.
- British Medical Research Council. Treatment of pulmonary tuberculosis with para-aminosalicylic acid and streptomycin. Br Med J 1949; 2: 1521-1525.
- Lehman J (1946), Para-aminosalicylic acid in the treatment of tuberculosis. Lancet; 5;1(6384):15.
- McLeay SC, Vis P, van Heeswijk RP, Green B. Population pharmacokinetics of Bedaquiline (TMC207), a novel anti-tuberculosis drug. Anti-Microbe Agents Chemother 2014;58:5315-24.
- Andries K, Villellas C, Coeck N, et al. (2014), Acquired resistance of Mycobacterium tuberculosis to Bedaquiline. PLoS One; 9(7):e102135.
- Schnippel K, Ndjeka N, Maartens G, et al. Effect of Bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med 2018;6: 699-706.
- Prasad, R., Gupta, N., Banka, A., 2018. Multi drug resistant tuberculosis/rifampicin-resistant tuberculosis: Principles of management. Lung India. 35:78-81.
- Raviglione M. (2006), XDR-TB: entering the post antibiotic era? Int J Tuberc Lung Dis; 10: 1185-1187.
- Zager EM, Mc Nerney R. (2008), Multi-drug- resistant tuberculosis. BMC Infectious Diseases; 8: 10. Available at http:// www.biomedcentral.com/1471-2334/8/10.
- Kant S,Lata H, Natu SM, Mishra AK, Verma NS. (2013), Diabetes mellitus with pulmonary tuberculosis–a double trouble. J Indian Med Assoc.; 111:187-91.
- Nagar V, Prasad P, Gour D, Singh AR, Pal D K. (2018), Screening for diabetes among tuberculosis patients registered under revised national tuberculosis control program, Bhopal, India. J Family Med Prim Care;7:1401-5
- Jadhav M, Khan T, Bhavsar C, Momin M, Omri A, (2019), Novel therapeutic approaches for targeting TB and HIV reservoirs prevailing in lungs Expert Opinion on drug delivery; 16: 687-699
- Srivastava K, Kant S, Verma AK (2015). Role of environmental factors in the transmission of Tuberculosis. Dynamics of Human Health 2 (4) ISSN-2382- 1019 (http://journalofhealth.co.nz)
- Srivastava K, Narain A, Bajpai J, Kant S. (2019); Respiratory Health Hazards in Women J Association Chest Physicians, 7:1-9.
- Surya Kant. Smoking or Health, the choice is yours. Proceedings Indian Medical Association, Lucknow 2004.
- Kant S, Verma SK, Anand SC, Vatsal P. Smoking and Pregnancy Obs & Gynae Today (2008); 7: 279-281.
- Dewan PK, Lal SS, Lonuroth K, Wares F, Uplekar M, Sahu S et al. (2006), Improving tuberculosis control through public-private collaboration in India. Literature review Brit Med J; 332: 574-578.
- Shrama SK, Surya K, Ryan H, Kapade S, Sachdeva KS, Singh AD, et al. (2017), Index-TB Guidelines: Guidelines on extrapulmonary tuberculosis for India. Indian J Med Res; 145; 448-463