A course of ‘cluster maintenance’ transcranial magnetic stimulation (TMS) can induce remission
Saxby Pridmore 1,2 Raimondo Bruno 3, Renee Morey 2, Yvonne Turnier-Shea 2, Marzena Rybak 2
1Discipline of Psychiatry, University of Tasmania, Hobart, Tasmania, Australia. 2TMS Unit, Saint Helens Private Hospital, Hobart, Tasmania. Australia. 3School of Psychological Sciences, University of Tasmania, Tasmania, Australia
Correspondence: Prof Saxby Pridmore: s.pridmore@utas.edu.au
Received: 13/2/2022; Accepted: 10/3/2022
Keywords: Transcranial magnetic stimulation; Major depressive disorder; Maintenance treatment; Relapse; Remission
[citation: Pridmore, Saxby; Bruno, Raimondo; Morey, Renee; Turnier-Shea, Yvonne; Rybak, Marzena. (2022). A course of ‘cluster maintenance’ transcranial magnetic stimulation (TMS) can induce remission. DHH, 9(1):http://www.journalofhealth.co.nz/?page_id=2728].
ABSTRACT
Objective: Patients suffering major depressive disorder (MDD) who come to transcranial magnetic stimulation (TMS) are prone to relapse. ‘Cluster maintenance’ TMS involves courses of 5 treatments delivered over 2.5-5 days separated by non-treatment periods. Our aim was to determine whether, when patients who have responded to acute TMS and have then received cluster maintenance TMS, but relapse despite this care, might achieve remission via a further 5 treatments. Method: This was a Quality Assurance/Clinical Audit study of a clustered maintenance service, over a one-year period. We studied the outcome of courses provided to patients who had recently relapsed/partially relapsed, according to the criteria of two rating scales (HAMD6 and CGI-S). In addition, a visual analogue scale for depression and the Sleep Quality Scale were administered. Results: Remission on both the HAMD6 and CGI-S occurred following 56% of courses, and remission on one occurred following 75% of courses. Large improvements were achieved on both subjective instruments. Conclusion: During a cluster maintenance TMS program, when relapse occurs, a further 5 treatment course will likely provide remission and improve well-being. Clustered maintenance programs ensure patients are treated regularly – this may explain, in-part, why a small number of treatments can produce high rates of remission.
Introduction
Major depressive disorder (MDD) is frequently a chronic condition featuring troublesome relapses. When psychotherapy, medication and ECT achieve remission they are often continued, in some form, in hope of preventing relapse. Patients who come to TMS have already failed other treatments and are therefore likely to relapse. Of patients suffering MDD who respond to acute TMS treatment, only 50% maintain in response/remission for 12 months [1].
TMS is a well-established treatment of acute MDD [2,3]. The use of TMS in the prevention/management of relapse is less well formalized. Various TMS protocols have been described. In one form, treatment sessions are continued at a less frequent rate – often once or twice a week for a month and then less frequently [4]. A second form, ‘cluster maintenance’, involves the administration of 5 TMS sessions over 2.5-5 days, initially at monthly intervals, with the period between courses increasing according to the clinical status of the patient [5]. Wilson et al [6] grouped these two protocols with ‘rescue courses’ (10 treatments) and coined the all-encompassing term, ‘preservation TMS’.
Given acute courses may extend to 36 treatments, the potency of short courses of 5 treatments in the treatment of relapse is uncertain. The aim of this investigation was to determine, when an acute course (20 treatments) of TMS has produced a remission and clustered maintenance has been implemented, but relapse has nevertheless ensued – whether a further course of 5 treatments might induce a remission. A positive result would support the claim that cluster maintenance TMS is a clinically useful service.
Method
We examined the records of a TMS Department of a private hospital, focusing on the cluster maintenance courses provided to inpatients over a one-year period. This was a Quality Assurance/Clinical Audit study approved by an institutional ethics committee. On admission patients gave written consent to their deidentified data being collected and analyzed. A pseudo-experimental ‘before and after’ design was possible where patients in our sample were their own control.
A course of treatment was 5 treatment sessions delivered once or twice daily (2.5-5 days) using a MagVenture device and figure-of-eight coil: 10Hz, 4 s trains, 110-120% RMT, 75 trains, applied to the dorsolateral prefrontal cortex.
The term ‘remission’ applies when symptoms are few or undetectable – it is objectively quantified using the six-item Hamilton Depression Rating Scale (HAMD6) as a score of <4 [7]. ‘Relapse’ refers to the return of the depressive episode after remission and is quantified as a HAMD6 score of > 7 [7,8]. The HAMD6 scores of 5 & 6, have been termed ‘Partial relapse’ [9,10]. The Clinical Global Impression – Severity (CGI-S) [11] is an established tool – remission is quantified as a score of < 2 and relapse is quantified as scores > 2 [12].
To explore the subjective experience of depression, we administered a visual analogue scale (VAS) [13]: a 10 cm line separating anchor points, “Not depressed” and “Most depressed possible” (termed ‘VAS-depression’). This was taken from a set of VASs which our group developed as a companion to the HAMD6 [14]. Finally, we administered a single-item sleep quality scale (SQS), an eleven-point instrument extending from “Terrible” to “Excellent” [15]. (Sleep difficulty is a common feature of MDD, but the HAMD6 does not measure it specifically.)
Only those courses provided to patients who had relapsed on both the HAMD6 (>4), and CGI-S (>2) were collected and analysed.
Results
176 courses were screened. 134 were provided to patients who had relapsed on both HAMD6 and CSI-S criteria. These were 50 separate individuals (7 males and 43 females) with an average age of 45 years (SD=15 years). Mean number of courses was 4 (SD=2, range 1-9).
Compared to pretreatment total scores (mean 8.3, SD=2.3) the mean post-treatment total HAMD6 score was reduced an average of 46.5% (mean 4.5, SD=2.2, t=22.2, p<0.001), with a large magnitude effect (Cohen’s d=1.92 [95%CI 1.63-2.20]). On the HAMD6, 57% of courses resulted in remission (94% of those in partial relapse, 48% of those in full relapse (Table 1).
Table 1: Summary of treatment outcomes of relapsed patients receiving maintenance TMS
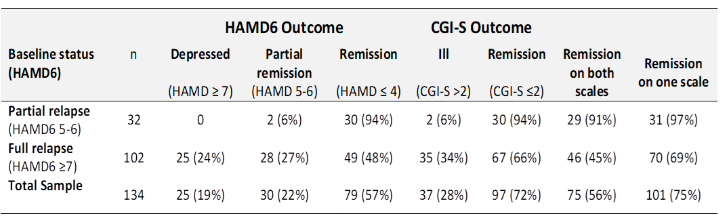
Similar changes were apparent on the other scales. The CGI-S significantly decreased by an average of 1.5 (95%CI 1.4-1.6) – this represents 38% reduction from baseline (Cohen’s d=1.87 [95%CI 1.59-2.15], p<0.001). On this measure, 72% of courses resulted in remission (94% of those in partial relapse at baseline, 66% of those in full relapse).
Thus, for individuals who had relapsed on both the HAMD6 and CGI-S, a further course of maintenance TMS induced remission on the HAMD6 in 57% of cases, and on the CGI-S in 72% cases. Remission on both tools occurred in 56% of courses, and on one or the other, in 75% of cases.
Mean VAS-depression ratings reduced by an average of 42% (average reduction 23.6, 95%CI 20.3-27.0), Cohen’s d=1.22 (95%CI 0.99-1.44), p<0.001.
SQS ratings improved by a mean of 2.0 (95%CI 1.6-2.4), Cohen’s d=0.79, 95%CI 0.59-0.98, p<0.001.
Thus, the positive effect on the objective instruments was supported by large improvements on the VAS-depression and the SQS, indicating enhanced subjective well-being.
Discussion and Conclusion
One of the limitations of the study may be that the study was limited to one institution and excluded comparison groups as in RCTs. However, this shortfall is compensated by (i) using real life clinical data, (ii) all patients in our sample were receiving TMS because they had failed other treatments, and (iii) in our ‘before and after’ study design individuals were used as their own control. This is useful because this approach allows exploring any change in outcome due to the treatment on the same individual as well as between individuals.
This is a study of patients who relapsed between courses of maintenance TMS. We found that for those relapsed on both the HAMD6 and CGI-S, the next course induced remission on the HAMD6 in 57% of cases, and the CGI-S in 72% cases. Remission on both tools occurred in 56% of courses, and on one of them in 75% of cases.
This positive effect was supported by large magnitude improvements on the VAS-depression and the SQS, indicating enhanced subjective well-being.
This report confirms and extends our earlier studies of TMS. We have demonstrated that when acute MDD has been successfully treated with a course of acute TMS (20 treatments), should relapse occur, a further acute course of TMS is likely to induce remission [2]. We have demonstrated that cluster maintenance TMS can prevent/delay relapse [16,17]. The current study demonstrates that should relapse occur during maintenance treatment, the small number of treatments (five) in the next maintenance course is likely to be sufficient to produce remission. This is a further indication of the value of TMS in difficult-to-manage, relapsing, treatment resistant depression.
That this small number of treatments can produce a high rate of remission is probably explained by the immediacy of treatment – clustered maintenance courses are scheduled at monthly (or slightly longer) intervals, thus relapses are treated early, before they become intractable. Also, it is remembered that all the patients in TMS maintenance programs have previously responded to TMS and are therefore likely to benefit from further TMS treatments – this was suggested as an important factor in our study demonstrating the success of second courses of acute TMS [2]. A question arises whether any form of treatment would benefit from the notion of clustering treatment. A larger and appropriately designed study is needed to address this question and other limitations of the present study.
References
- Senova S, Cotovio G, Pascual-Leone A, Oliveira-Maia A. (2019) Durability of antidepressant response to repetitive transcranial magnetic stimulation: systematic review and meta-analysis. Brain Stimul. 12(1): 119-128. https://doi: 10.1016/j.brs.2018.10.001.
- Pridmore S, Erger S, May T. (2019) Second courses of transcranial magnetic stimulation (TMS) in major depressive episodes for initial responders and non-responders. Malays J Med Sci 26(3): 102–109. https://doi.org/10.21315/ mjms2019.26.3.8
- Fitzgerald P, George M, Pridmore S (2021) The evidence is in: Repetitive transcranial magnetic stimulation is an effective, safe and well-tolerated treatment for patients with major depressive disorder. Aust N Z J Psychiatry. Aug 28;48674211043047. https://doi: 10.1177/00048674211043047.
- O’Reardon, J, Blumner, K, Peshek, A, Pradilla R, Pimiento P. (2005) Longterm maintenance therapy for major depressive disorder with rTMS. J Clin Psychiatry 66(12):1524-8. https://doi: 10.4088/jcp.v66n1205.
- Fitzgerald P, Grace N, Hoy K, Bailey M, Daskalakis Z. (2013) An open label trial of clustered maintenance rTMS for patients with refractory depression. Brain Stimul 6(3): 292–297. http://doi: 10.1016/j.brs.2012.05.003.
- Wilson S, Croarkin P, Aaronson S, et al. (2022) Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord 296 :79-88.http://doi: 10.1016/j.jad.2021.09.040.
- Bech P, Lunde M, Bech-Andersen G, et al. (2007) Psychiatric outcome studies (POS): Does treatment help the patients? A Popperian approach to research in clinical psychiatry. Nord J Psychiatry 61(Suppl 46): 4-34. http://doi: 10.1080/08039480601151238.
- Bachner Y, O’Rourke N, Goldfracht M, et al. (2013) Psychometric properties of responses by clinicians and older adults to a 6-item Hebrew version of the Hamilton Depression Rating Scale (HAMD6). BMC Psychiatry 13: 2. http://doi: 10.1186/1471-244X-13-2.
- Rush A, Kraemer H, Sackheim H, et al. (2006) Report by the ACNP Taskforce on response and remission in major depressive disorder. Neuropsychopharmacology 31(9): 1841-1853. http://doi: 10.1038/sj.npp.1301131.
- Paykel E (2008) Partial remission, residual symptoms, and relapse into depression. Dialogues Clin Neurosci 10(4): 431-437. http://doi:10.31887/DCNS.2008.10.4/espaykel.
- Guy W. (1976) ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration.
- Bandelow B, Baldwin D, Dolberg O, et al. (2006) What is the threshold for symptomatic response and remission for major depressive disorder, panic disorder, social anxiety disorder, and generalized anxiety disorder? J Clin Psychiatry 67(9): 1428–1434. https://doi.org/10.4088/JCP.v67n0914
- Cowdry R, Gardner D, O’Leary K, et al. (1991) Mood variability: a study of four groups. Am J Psychiatry 148(11): 1505-1511. http://doi: 10.1176/ajp.148.11.1505.
- May T, Pridmore S. (2020) A visual analogue scale companion for the six-item Hamilton Depression Rating Scale, Australian Psychologist 55(1): 3-9. https://doi.org/10.1111/ap.12427
- Snyder E, Cai B, DeMuro C, Morrison M, et al. (2018) A new single-item sleep quality scale: results of psychometric evaluation in patients with chronic primary insomnia and depression. J Clin Sleep Med 14(11): 1849-1857.http://doi: 10.5664/jcsm.7478.
- Pridmore S, Erger S, Rybak M, et al. (2018) Early relapse (ER) transcranial magnetic stimulation (TMS) in treatment resistant major depression. Brain Stimul 11(5): 1098-1102. http://doi: 10.1016/j.brs.2018.05.013.
- Pridmore S, May T. (2018) Relapse prevention (RP) TMS. Brain Stimul 11(6): 1391–1392. http://doi: 10.1016/j.brs.2018.08.004.